 28:22
28:22
 54:27
54:27
 52:18
52:18
 55:06
55:06
 1:39:26
1:39:26
 2:10:25
2:10:25
 1:26:42
1:26:42
 16:47
16:47
 56:55
56:55
 23:06
23:06
 3:22:50
3:22:50
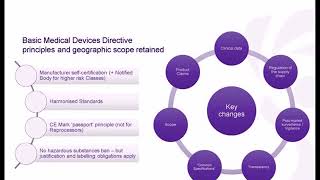 1:06:28
1:06:28
 1:03:39
1:03:39
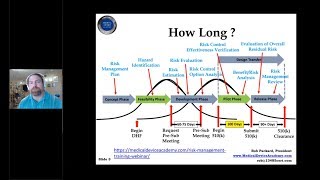 1:34:30
1:34:30
 28:21
28:21
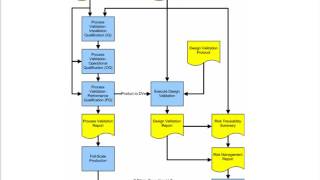 1:28:00
1:28:00
 1:36:58
1:36:58
 56:14
56:14
 24:26
24:26


